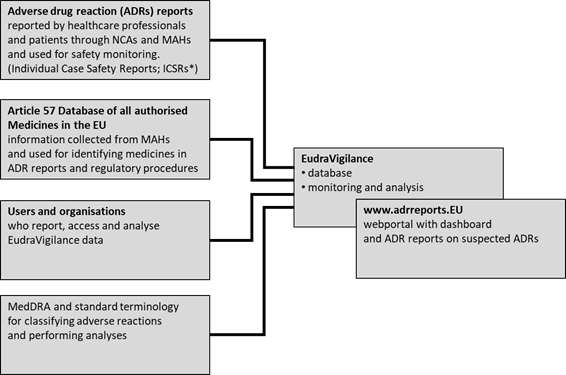
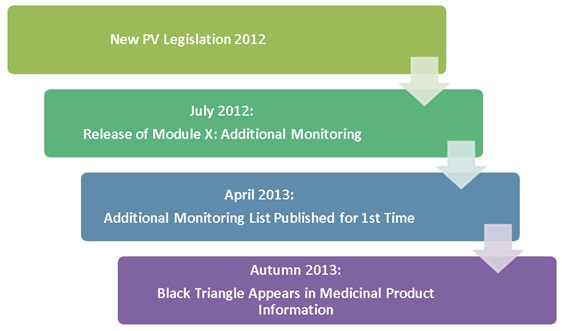
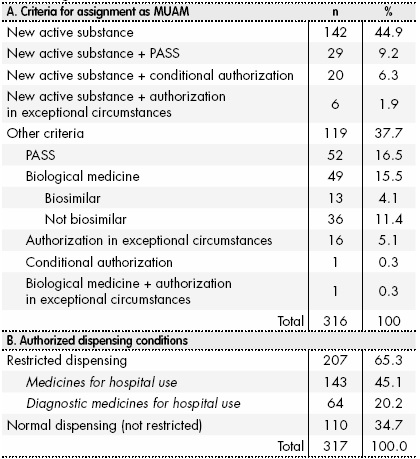
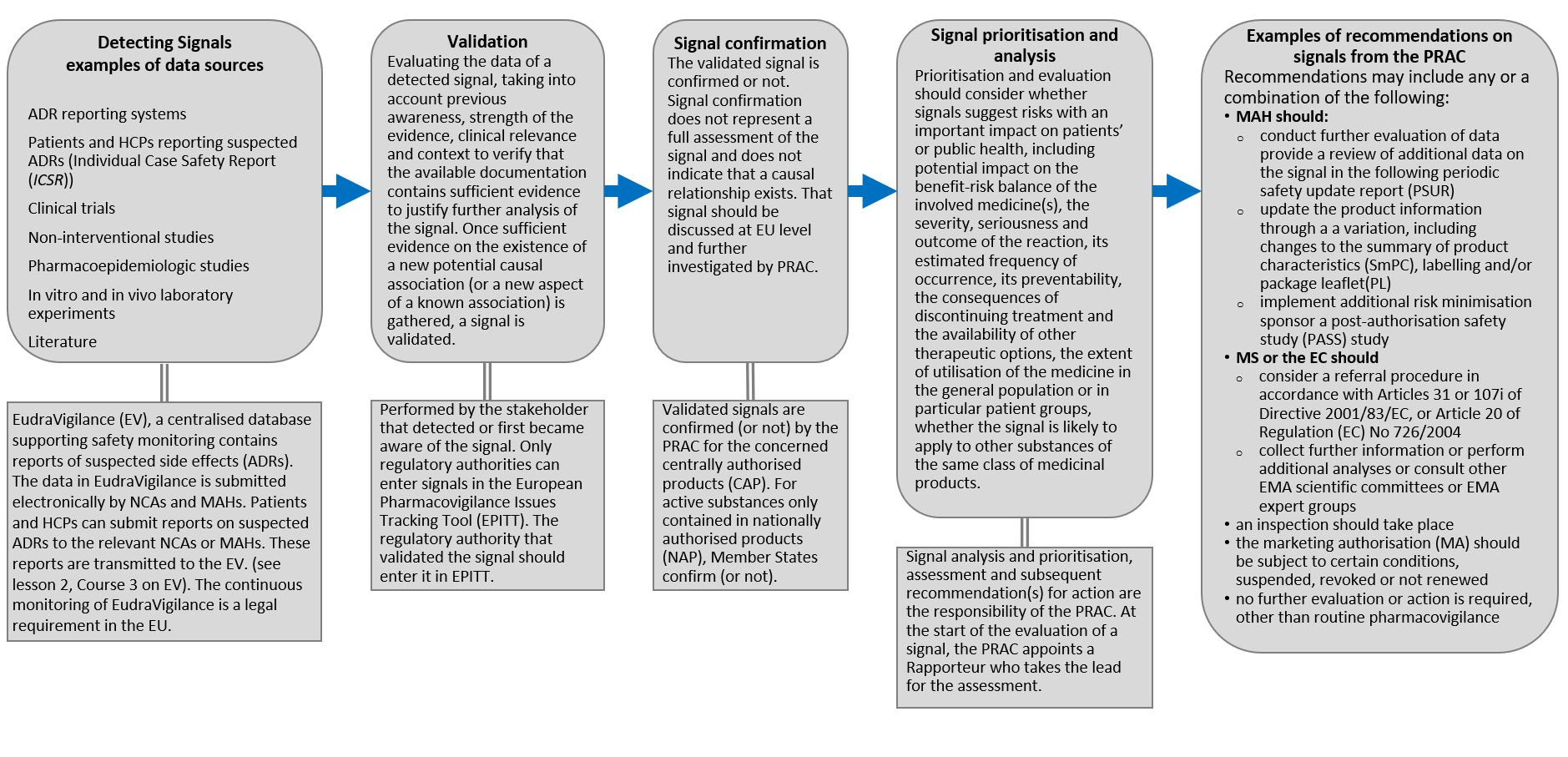
Improving the Safety of Medicines in the European Union: From Signals to Action - Potts - 2020 - Clinical Pharmacology & Therapeutics - Wiley Online Library

Risk-Based Biologics: CMC Flexibilities in the EU Regulatory System - BioProcess InternationalBioProcess International

PDF) Does additional monitoring status increase the reporting of adverse drug reaction s ? An interrupted time series analysis of EudraVigilance data

Evaluation of quantitative signal detection in EudraVigilance for orphan drugs: possible risk of false negatives | Semantic Scholar